About Water
A major reason why the club has
been successful in hatching
Atlantic salmon eggs lies in the quality of our water supply. We
take it for granted but life is dependant on the quality of water
we have access to. Since water is so important to us and to all
forms of life, it may be worthwhile to discuss some of the
properties of water.
Water is unusual in that it is
liquid under normal conditions.
A study of other hydrides suggest that it should be a gas. Water
is the only natural substance found in each of its natural states
(solid, liquid, gas) on Earth. Water is called the universal
solvent, dissolving many other chemical substances, such as
salts, sugars, acids, alkialis, some gases and many organic
substances. Oxygen dissolves in water and is essential for
aquatic life. Cold water can contain more oxygen than warm water.
Because water is polar, it
sticks to itself (cohesion). Water
has a high surface tension and insects that have to penetrate the
water surface struggle to do so. This is the reason why our dry
flies and the insects we are trying to imitate "float"
on the water. Actually, mayflies "stand" on the surface
of water.
Water is also adhesive (sticks
to other things) because of its
polar nature. Cohesion and adhesion are responsible for capillary
action which allows water to flow up plants and trees from their
roots. In capillary action, water adheres to the walls of
structures in plants and because of cohesion more water is drawn
up. The process repeats until the gravity is strong enough to
counteract the adhesive forces. This allows plant life to prosper
on Earth.
Click on image for bigger image |
As water cools it gets
more dense until it reaches 4 degrees C or 39 degrees F. If cooled
further, water becomes less dense. It is this reason why ice forms on
top of water, rather than on the bottom. When a pond freezes over, the
water at the bottom of the pond will be warmer than the water just
under the ice. Otherwise ponds and lakes would be frozen solid and
aquatic life would be jeopardized.
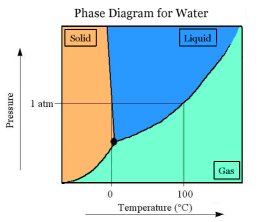
Water turns to ice at 32
degrees F or 0 degrees C at normal air pressure. Water at normal air
pressure cannot stay liquid at temperatures less than 32 degrees F. In
order for ice to form, the water must lose a lot of heat. Water at 32
degrees holds twice the amount of heat as ice at the same temperature.
Conversely, to melt ice, it must absorb a lot of heat.
|
Water also has the second
highest specific heat of any known
chemical. The specific heat is the amount of heat
per unit mass required to raise the temperature
by one degree Celsius. In
other words more heat is required to
heat water than an equal amount of earth. When scientists talk
about the oceans warming and the polar ice pack melting, think
about the amount of heat required. There should be no doubt about
Global Warming as an issue.
Source: Wikipedia
